|
9th March 2009
Since November 2007 there has been a significant change in the prescribing rate of diclofenac (a decline of about 5% of total NSAID items per year) and naproxen (an increase of about 4% of total NSAID items per year). This change in prescribing rates coincides with our initiatives here at the NPC to encourage medication review and more appropriate prescribing of NSAIDs, taking into account their CV and GI risks. Further impetus has been given to this by the recent Drug Safety Update from the MHRA which highlighted two further studies around CV risk of NSAIDs.
Action
The trend for reduced prescribing of diclofenac, mostly in favour of naproxen, is appropriate given the cardiovascular (CV) risk associated with its use, which is similar to that of coxibs. However, prescribing of non-steroidal anti-inflammatory drugs (NSAIDs) as a whole, and diclofenac in particular, remains at a relatively high level. This is particularly apparent in some PCT areas and also in some individual practices. Prescribers should continue to follow our previous recommendations given in MeReC Extra 30 when considering the prescribing of NSAIDs:
- Where NSAIDs are required, prescribing should be based on the safety profiles of individual NSAIDs and on individual patient risk factors. All NSAIDs should generally be used at the lowest effective dose and for the shortest period of time necessary to control symptoms.
- The ideal anti-inflammatory prescribing choice will vary from patient to patient, depending on individual risk factors, therapeutic response, patient preference, and the patient’s attitude to the risk of adverse events.
- Low-dose ibuprofen (≤1200mg per day) is an appropriate first choice NSAID in view of its low risk of gastrointestinal (GI) and CV side effects.
- Low-dose ibuprofen or naproxen 1000mg per day would appear more appropriate than other NSAIDs for patients in whom CV risk is a significant consideration in decision making.
- Consider prescribing a proton pump inhibitor (PPI) with any NSAID to reduce the risk of adverse GI effects, particularly in those who are at high GI risk (includes anybody aged 65 years or older) and long-term NSAID users. PPIs should be prescribed routinely with NSAIDs for people with osteoarthritis or rheumatoid arthritis according to NICE clinical guidelines 59 and 79).
- Although coxibs are associated with a lower risk of GI side effects than traditional NSAIDs, there is no good evidence to support the use of coxibs alone ahead of traditional NSAIDs co-prescribed with a PPI. Coxibs also have a higher CV risk than ibuprofen ≤1200mg per day or naproxen 1000mg per day.
What is the background to this?
Although the CV risk associated with coxibs were already well recognised, in June 2006, a meta-analysis of randomised controlled trials by Kearney et al raised concerns about the CV safety of non-selective (also termed traditional or standard) NSAIDs. It was suggested that high doses of diclofenac and ibuprofen, but not naproxen, were associated with an increased risk of CV events. In October 2006, a review of the CV safety of selective and non-selective NSAIDs, reported by the Commission on Human Medicines, identified that diclofenac (especially 150mg per day) was associated with a small increased thrombotic risk similar to that of etoricoxib and possibly other coxibs. High-dose ibuprofen (e.g. 2400mg per day) also appeared to be associated with a small increased thrombotic risk, whereas low-dose ibuprofen (e.g. ≤1200mg per day) and naproxen (1000mg per day) were not.
Two recent epidemiological studies, reviewed in the February 2009 edition of Drug Safety Update, provide further evidence that a thrombotic CV risk may apply to all NSAID users, regardless of their baseline risk, with the greatest concern being for chronic users of high doses (in particular coxibs and diclofenac).
As pointed out in MeReC Extra 30, taking into account the high prescribing volume of diclofenac (approximately 2 million items per quarter in primary care in England) and the increased thrombotic risk being similar to that of coxibs (3 per 1000 patients per year), the prescribing of diclofenac could be associated with up to 2000 extra or premature CV events in England each year, compared with no treatment.
NPC initiatives
In the second half of 2007, in view of the CV risks associated with the high prescribing volume of diclofenac, here at the the National Prescribing Centre (NPC) we initiated a number of activities to raise awareness of the safety issues around the use of NSAIDs, and to encourage review of NSAID prescribing and more appropriate choice of NSAIDs for patients based on an assessment of their CV and GI risk. In September 2007 a programme of NPC funded workshops was initiated, supported by educational materials produced by the NPC and MeReC Extra 30 which was published in November 2007.
What does the prescribing data show?
From December 2005 to November 2008 there was a statistically significant reduction of NSAID items per year (P=0.017), equivalent to a mean reduction over the three-year period of 5.6%.
In the six-months from June to November 2007 there was a decrease of 4% in the proportion of diclofenac prescribed relative to the total number of NSAID items prescribed compared with the proportion prescribed in the same six-month period one year earlier (from 46.3% to 42.3%). This was accompanied by a similar increase in the proportion of naproxen prescribed (from 7.8% to 11.7%). Although there were some changes in the quantities of other NSAIDs prescribed, the absolute changes were very much smaller, with the largest being ibuprofen (an increase of about 1%).
Analysis of the changes in the proportions of NSAIDs prescribed each month before and after November 2007 identified a statistically significant change in rates of prescribing of both diclofenac and naproxen (both P<0.0001). Before November 2007 there was a significant increase in the rate of diclofenac prescribing (0.07%/month, P=0.003), whereas after November 2007 there was a significant decrease (0.33%/month, P=0.0002) (Figure 1). There was a significant increase in the rate of naproxen prescribing both before (0.08%/month, P<0.0001) and after (0.40%/month; P<0.0001) November 2007. These changes are equivalent to a shift in prescribing rates of –4.9%/year for diclofenac and +3.9%/year for naproxen, relative to the total quantity of NSAID items prescribed. There was no statistically significant change in the rates of prescribing of ibuprofen before and after November 2007 (P=0.06 and P=0.97 respectively). However, there was an indication of a small upward shift (about 1%) in the prescribing of ibuprofen after November 2007 compared with the period before November 2007 (P=0.02).
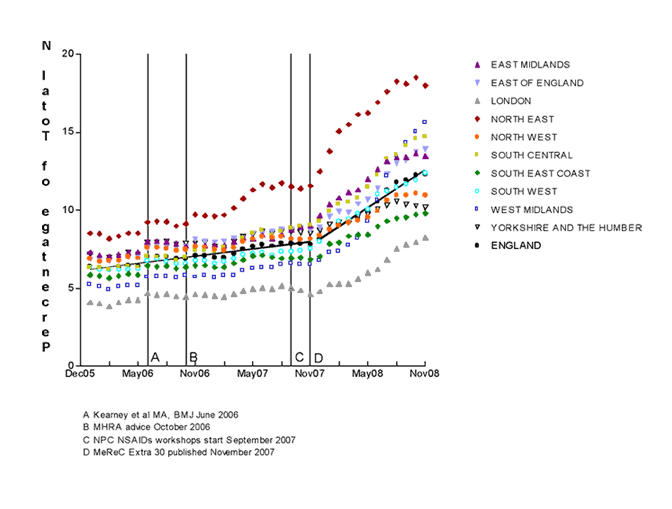
Absolute proportions and changes in the rates of prescribing of diclofenac and naproxen after November 2007 varied widely among individual strategic health authorities (SHAs). (see Figures 2 and 3). However, only in one case (diclofenac in Yorkshire and the Humber SHA) was there no statistically significant shift in the rates of prescribing for diclofenac (decrease) or naproxen (increase) identified before and after November 2007.
Figure 1. Trends in the prescribing of diclofenac, naproxen and ibuprofen in England (December 2005 to November 2008).
The lines represent the linear regression lines for the periods before and after November 2007.
Figure 2. Prescribing of diclofenac in England (December 2005 to November 2008).
The lines represent the linear regression lines for the proportion of diclofenac prescribed in England overall before and after November 2007.
Figure 3. Prescribing of Naproxen in England (December 2005 to November 2008).
The lines represent the linear regression lines for the proportion of naproxen prescribed in England overall, before and after November 2007.
Acknowledgments:
The data used for the analysis reported in this blog was kindly supplied by the NHS Business Services Authority.
Feedback
Please comment on this blog in the NPC discussion rooms, or using our feedback form